
In a Fischer projection, the carbon atoms of a sugar molecule are connected vertically by solid lines, while carbon-oxygen and carbon-hydrogen bonds are shown horizontally. While organic chemists prefer to use the dashed/solid wedge convention to show stereochemistry, biochemists often use drawings called Fischer projections and Haworth projections to discuss and compare the structure of sugar molecules.įisher projections show sugars in their open chain form.

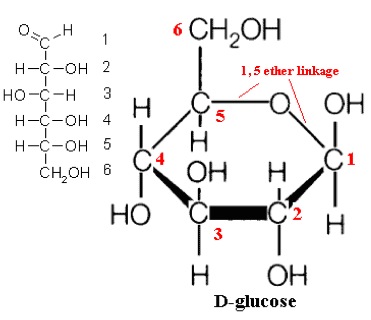
When reading the chemical and biochemical literature, you are likely to encounter several different conventions for drawing molecules in three dimensions, depending on the context of the discussion.


 0 kommentar(er)
0 kommentar(er)
